I CH2 CH - Cl. Assessing the Relative Importance of Resonance Structures.
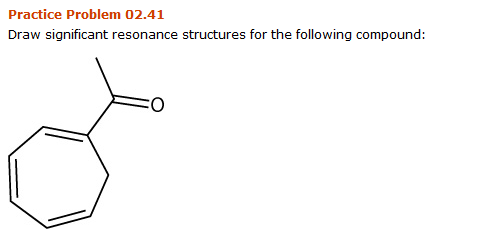
Solved Practice Problem 02 41 Draw Significant Resonance Chegg Com
All of the following are significant resonance structures of the benzyl carbocation EXCEPT.

. Draw significant resonance structures for the following compound. Organic Chemistry 222 Fall 2016 Recitation Problem Set 2 Sep 13th 11 a Use resonance structures to help you identify all sites of low electron density d in the following. Draw in any missing lone pairs as needed and use proper arrows.
An allylic lone pair. Circle and name the functional groups present in the following molecules. We can obtain the following resonance structures by following the same steps as mentioned above.
SH 0 3 HO O O HN O NH2 3. 1 Do not exceed the octet on 2nd-row elements. For each of the following compounds draw all of the significant resonance structures.
Draw significant resonance structures for the following compound. Organic Chemistry as a Language. 2 Add curved arrows to both structures to show the delocalization of electron pairs.
Draw all significant resonance structures of the following compounds and label the most significant contributors. Draw and rank all significant resonance forms for the following compound. Rules for drawing resonance structures.
Include lone pairs and charges in your structure. NH2 Step 1 Get help answering Molecular Drawing questions x Your answer is incorrect. 1 Draw the other significant resonance contributor for the following compound.
What is the IUPAC name for the following compound. They are in fact two differe SolutionInn. Please provide the answers to the question Q.
Draw all significant resonance structures for each of the following compounds. Therefore we can draw resonance structures for O 3 molecule as follows. Click hereto get an answer to your question Draw the resonance structures of the following compounds.
As a result the C-H bond is almost completely broken in the transition state and the carbon atom has significant radical character Figure 1014. A Draw the best Lewis structure for each of the following compounds. A lone pair next to a pi bond.
Draw all significant resonance structures of the following compounds and label the most significant contributorls. Draw in anyr missing lone pairs as needed and use proper arrows. I Rb 2 O.
20 Which of the following violates the rules for curved arrows. Ii CH2 CH - CH CH2 iii CH2 CH - H C O Solve Study Textbooks Guides. Draw resonance structures for the conjugate base that is produced when each of the following compounds is treated with sodium ethoxide.
Transcribed image text. 43 MNHZ O 0. There are also several patterns for drawing resonance structures of radicals.
Resonance structures and expanded valence shells where appropriate. BIn the Lewis structure shown here the central atom represented as A is an. Answer to The following two drawings are resonance structures of one compound.
O First add curved arrow s to show the resonance using the following pattern. N H N H N H a N N N b N N c C N O C N O d S O S O e f h O O i C N C N C N C N j k O OH l H. O Step 1 Your answer is incorrect First add curved arrows to show the resonance using the following patterns a pi bond between two atoms of differing electronegativity Modify the second structure given to draw the new resonance structure.
Include lone pairs of electrons formal charges and hydrogen atoms. Include lone pairs and charges in your structure. Resonance Structures are a representation of a Resonance Hybrid which is the combination of all resonance structures.
First add curved arrows to show the resonance using the following pattern. The second-row elements C N O F can only handle up to. Two must-follow rules when drawing resonance structures.
Use resonance structures to help you identify all sites of high electron density δ- in the following compound. Draw all significant resonance structures for the following compound. Modify the second structure given to draw the.
The resonance structure with the Formal Charge closest to zero is the most accepted structure however the correct Lewis structure is actually a combination of all the resonance structures and is not solely describe as one. But the following two drawings are not resonance structures. Draw the Lewis Structure Resonance.
Students also viewed these Sciences questions Consider the structure of. Draw resonance structures for following compounds Show electron shift using curved arrow notation Draw the resonance structures for the following compounds. Draw all 8 constitutional isomers of C3H50.
Practice Problem 0252e Draw all significant resonance structures for the following compound. 2 Do not break single bonds. Draw all significant resonance structures for each of the following compounds.
Draw significant resonance structures for the following compound. A b c Draw the contributing resonance structures for the following anion and rank them. Modify the second structure given to draw the new resonance structure.
Briefly explain your rankings. It gives the following structure and has a multiple bonds and an adjacent atom with one lone pair of electrons. IMAGE IS NOT AVAILABLE TO COPY Answer.
Ii The compound formed between calcium and the chlorite ion ClO 2.

Draw Significant Resonance Structures For The Following Compound Which Of This Is Are Most Significant Resonance Structures Study Com

Oneclass Which Of The Following Is The Most Significant Resonance Structure
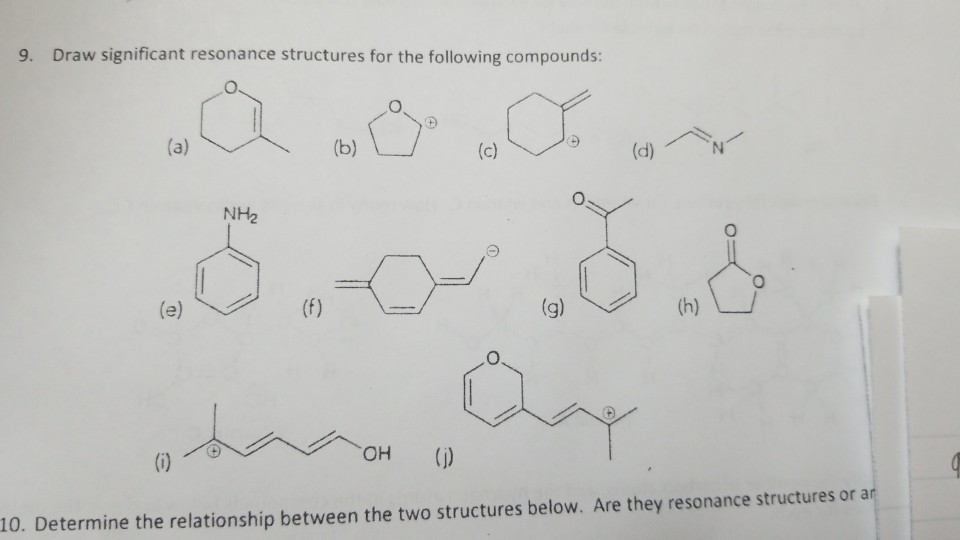
Solved 9 Draw Significant Resonance Structures For The Chegg Com

Solved Practice Problem 02 41 Draw Significant Resonance Chegg Com

Draw Significant Resonance Structures For The Following Compound Which Of This Is Are Most Significant Resonance Structures Study Com
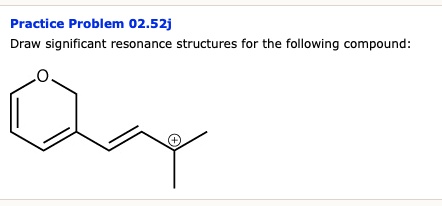
Solved Practice Problem 02 52j Draw Significant Resonance Structures For The Following Compound

Solved 2 41 Draw Significant Resonance Structures For The Chegg Com

Solved Draw Significant Resonance Structures For The Chegg Com
0 comments
Post a Comment